Rationally designed an innovative proximity labeling near-infrared fluorogenic probe for imaging of peroxynitrite in acute lung injury
Author links open overlay panel,
,
,
,
,
,
- a
- Key Laboratory of Emergency and Trauma of Ministry of Education, Department of Radiotherapy, The First Affiliated Hospital of Hainan Medical University, Hainan Medical University, Haikou 571199, China
- b
- Key Laboratory of Hainan Trauma and Disaster Rescue, Key Laboratory of Haikou Trauma, Engineering Research Center for Hainan Bio-Smart Materials and Bio-Medical Devices, College of Emergency and Trauma, Hainan Medical University, Haikou 571199, China
Received 8 April 2024, Revised 30 May 2024, Accepted 2 June 2024, Available online 3 June 2024.
https://doi.org/10.1016/j.cclet.2024.110082
Chinese Chemical Letters Available online 3 June 2024, 110082
ABSTRACT
Acute lung injury (ALI) is a serious clinical condition with a high mortality rate. Oxidative stress and inflammatory responses play pivotal roles in the pathogenesis of ALI. ONOO− is a key mediator that exacerbates oxidative damage and microvascular permeability in ALI. Accurate detection of ONOO− would facilitate early diagnosis and intervention in ALI. Near-infrared fluorescence (NIRF) probes offer new solutions due to their sensitivity, depth of tissue penetration, and imaging capabilities. However, the developed ONOO− fluorescent probes face problems such as interference from other reactive oxygen species and easy intracellular diffusion. To address these issues, we introduced an innovative self-immobilizing NIRF probe, DCI2F-OTf, which was capable of monitoring ONOO− in vitro and in vivo. Importantly, leveraging the high reactivity of the methylene quinone (QM) intermediate, DCI2F-OTf was able to covalently label proteins in the presence of ONOO−, enabling in situ imaging. In mice models of ALI, DCI2F-OTf enabled real-time imaging of ONOO− levels and found that ONOO− was tightly correlated with the progression of ALI. Our findings demonstrated that DCI2F-OTf was a promising chemical tool for the detection of ONOO−, which could help to gain insight into the pathogenesis of ALI and monitor treatment efficacy.
Graphical Abstract
An innovative self-immobilizing NIRF probe DCI2F-OTf was capable of monitoring ONOO− in vitro and in vivo. DCI2F-OTf was able to covalently label proteins in the presence of ONOO−, enabling in situ imaging. In mice models of ALI, DCI2F-OTf enabled real-time imaging of ONOO− levels and found that ONOO− was tightly correlated with the progression of ALI.
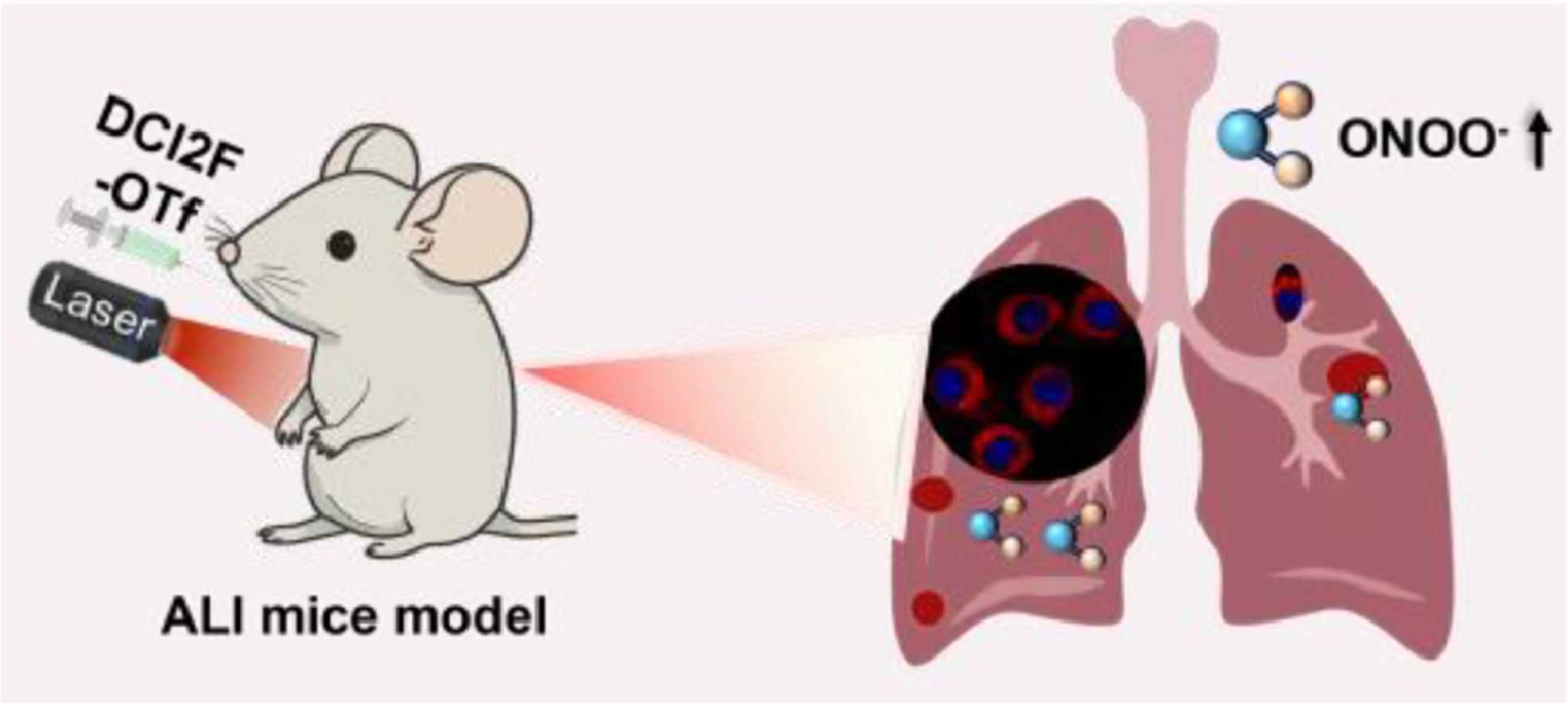
ONOO-作为信号分子参与细胞信号转导和各种生理过程,包括基因表达调控和细胞凋亡。一方面,升高的ONOO-会破坏肺部抗氧化防御系统,加剧氧化应激和细胞损伤;另一方面,增加的ONOO-水平会显著增强肺微血管壁的通透性,最终损害肺功能。因此,开发用于检测ONOO-的新型工具以准确诊断ALI将有助于及时跟踪疾病进程,进行早期干预,提高患者生存率。现有的ONOO-荧光探针面临两个主要挑战:(1)易受高浓度活性氧(如过氧化氢)的干扰;(2)容易从反应位点扩散,降低成像精度和信噪比。因此,设计ONOO-激活的、邻近蛋白捕获的近红外荧光探针在细胞或活体水平上具有重要意义。
急性肺损伤(ALI)是一种严重的临床病症,具有较高的死亡率。氧化应激和炎症反应在ALI的发病机制中起着关键作用。过氧亚硝酸盐(ONOO-)是加剧ALI中氧化损伤和微血管通透性的关键介质。准确检测ONOO-有助于ALI的早期诊断和干预。目前开发的ONOO-荧光探针面临着其他活性氧物质干扰和易于细胞内扩散的问题。为了解决这些问题,我们设计了一种新型邻近标记近红外荧光探针DCI2F-OTf,该探针能够在体内外监测ONOO-。重要的是,利用亚甲基醌中间体的高反应性,DCI2F-OTf能够在ONOO-存在下共价标记蛋白质,从而实现原位成像。在ALI小鼠模型中,DCI2F-OTf实现了ONOO-的实时成像,发现ONOO-与ALI的进展密切相关。研究结果表明,DCI2F-OTf是一种有前途的化学工具,将有助于理解ALI的发病机制和监测治疗效果。
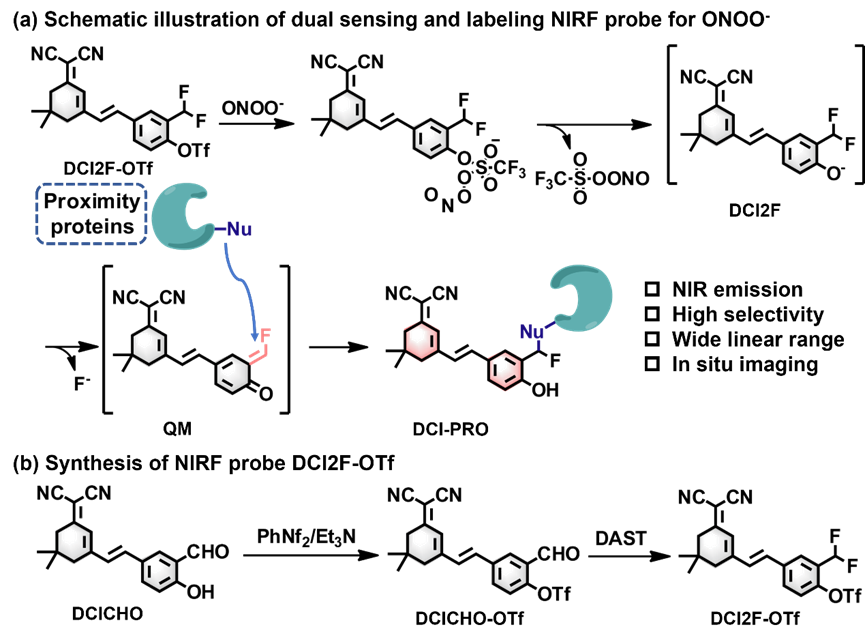
Scheme 1. (a) Schematic illustration of dual sensing and labeling NIRF probe DCI2F-OTf for ONOO-. (b) Synthesis of NIRF probe DCI2F-OTf. 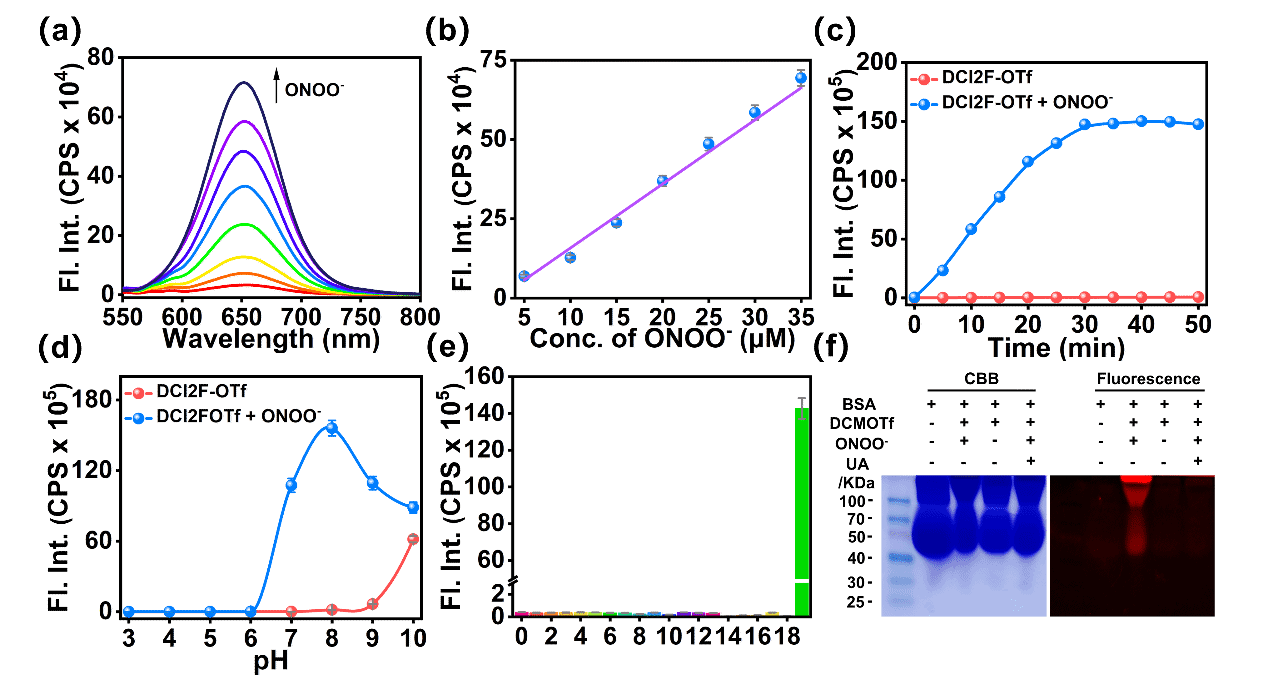
Figure 1. Fluorescence response of DCI2F-OTf (10 μM) to ONOO- excited at 490 nm. (a) Visualization of the fluorescence changes of DCI2F-OTf during titration with increasing ONOO- (0 - 35 μM) concentrations. (b) Linearity of the fluorescence intensity value of DCI2F-OTf at 652 nm with varied concentrations of ONOO- (5 - 35 μM). (c) Time-dependent fluorescence response of DCI2F-OTf before and after the addition of ONOO- (70 μM) at 37 °C recorded every 5 min. (d) The fluorescence intensities at 652 nm of DCI2F-OTf in the absence or presence of ONOO- (70 μM) over pH range from 3.0 to 7.0. (e) Values of fluorescence intensities of DCI2F-OTf treated with different competitive biological agents: 1. blank, 2. Na+ (500 μM), 3. K+ (500 μM), 4. Ser (200 μM), 5. Leu (200 μM), 6. Arg (200 μM), 7. S2O32- (100 μM), 8. HSO3- (100 μM), 9. H2S (100 μM), 10. Cys (100 μM), 11. GSH (1 mM), 12. NO2- (100 μM), 13. NO3- (100 μM), 14. NO (100 μM), 15. •OH (100 μM), 16. O21 (100 μM), 17. H2O2 (100 μM), 18. O2•- (100 μM), 19. HOCl (100 μM), 20. ONOO- (70 μM). (f) SDS-PAGE analysis of BSA (5 mg/mL) after incubation with DCI2F-OTf + ONOO-, DCI2F-OTf, DCI2F-OTf + ONOO- + UA in PBS at 37 ℃ for 30 min. Left: Coomassie brilliant blue staining; right: fluorescence imaging. λex/λem = 490/650 nm.
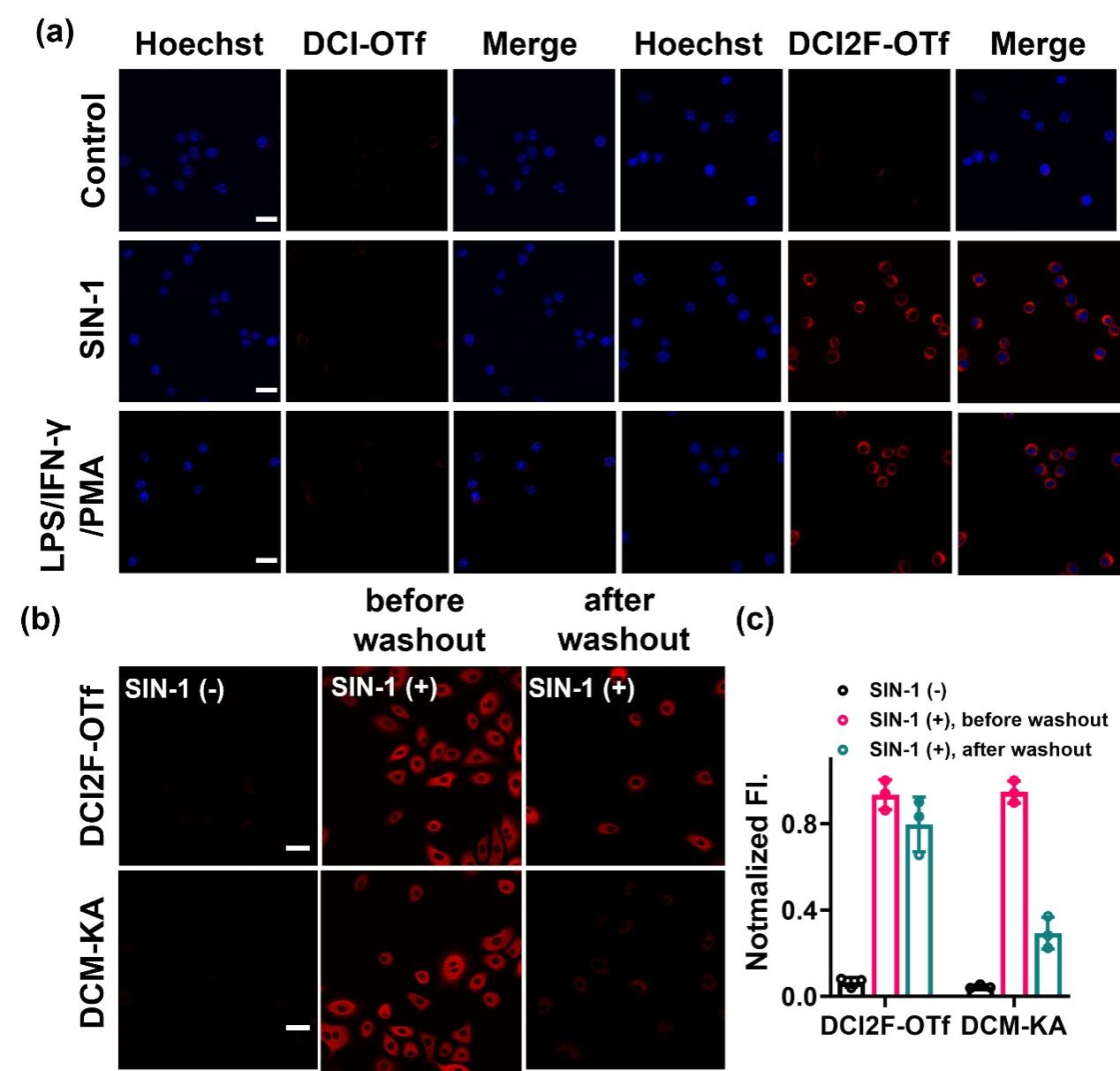
Figure 2. (a) RAW264.7 cells were pretreated with SIN-1 (200 μM, 30 min) or stimulated with LPS (1.0 μg/mL), IFN-γ (100 ng/mL) for 12 h, PMA (10 nM) for 30 min, and then incubated with DCI-OTf (10 μM, 30 min) or DCI2F-OTf (10 μM, 30 min), respectively. The red channel was collected in the range of 600-700 nm (λex = 488 nm) and the Hoechst channel was collected in the range of 430-480 nm (λex = 405 nm). (b) A549 cells were pretreated with SIN-1 (200 μM, 30 min), followed by incubation with DCI2F-OTf (10 μM, 30 min) or DCM-KA (10 μM, 30 min), before or after washout with fresh DMEM (three times, each for 6 min). (c) Normalized fluorescence intensity plots of DCI2F-OTf and DCM-KA labeled cells in (b) (n = 3). Scale bar: 50 μm.
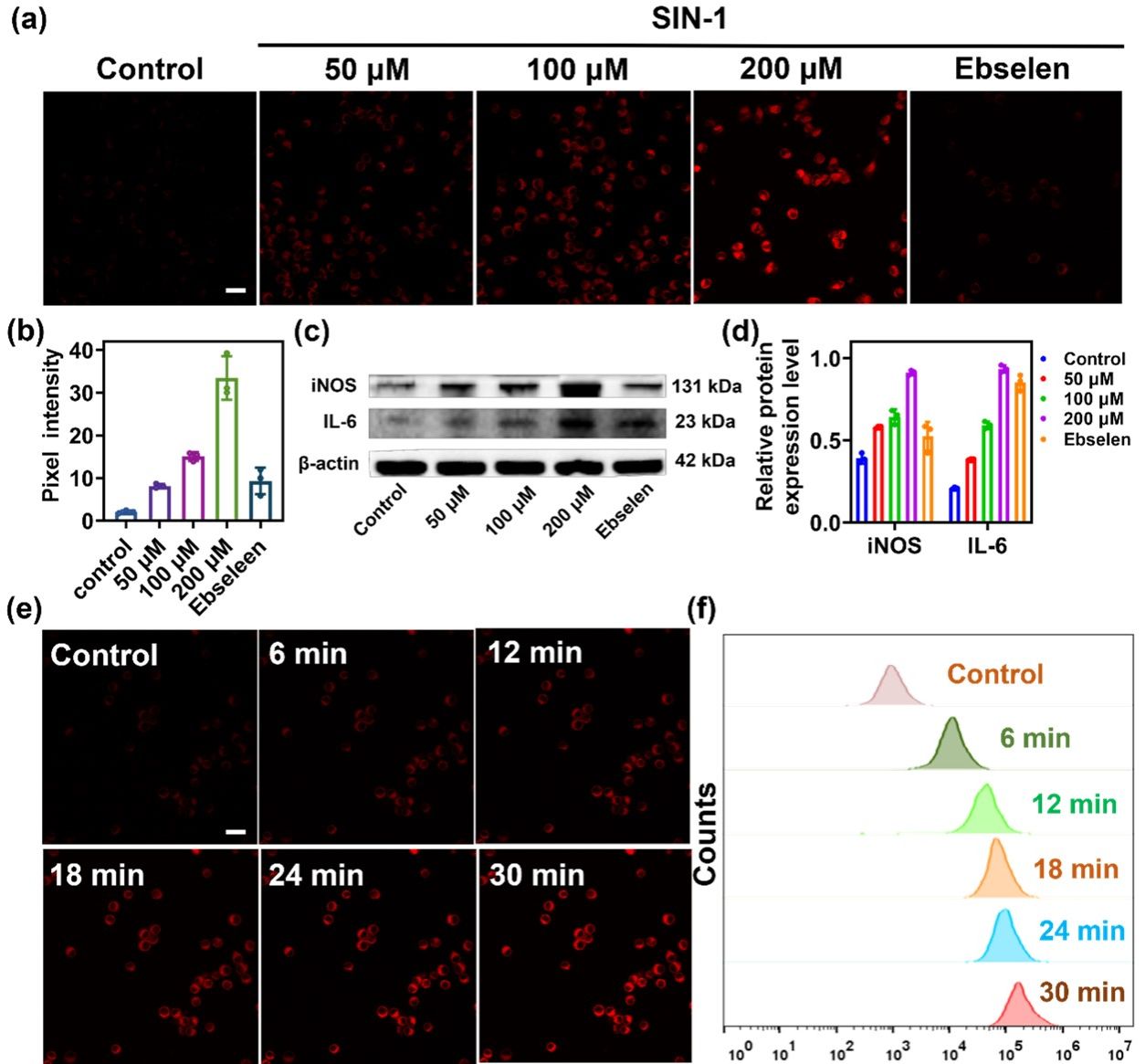
Figure 3. (a) RAW264.7 cells pretreated with different concentrations of SIN-1 (0, 50, 100, 200 μM), SIN-1 (200 μM) plus ebselen (200 μM) for 30 min were incubated with DCI2F-OTf (10 μM) for 30 min. (b) The pixel intensity of DCI2F-OTf labeled cells in (a). (c) The protein levels of iNOS and IL-6 were detected by western blot analysis. (d) The relative protein expression level of iNOS and IL-6 in (c). (e) RAW264.7 cells were pretreated with SIN-1 (200 μM, 30 min), and then incubated with DCI2F-OTf (10 μM) for 0 - 30 min. (f) Flow cytometry analysis of DCI2F-OTf (10 μM) labeled SIN-1 (200 μM, 30 min) pretreated cells. Error bars represent s.d., n=3. Scale bar: 50 μm.
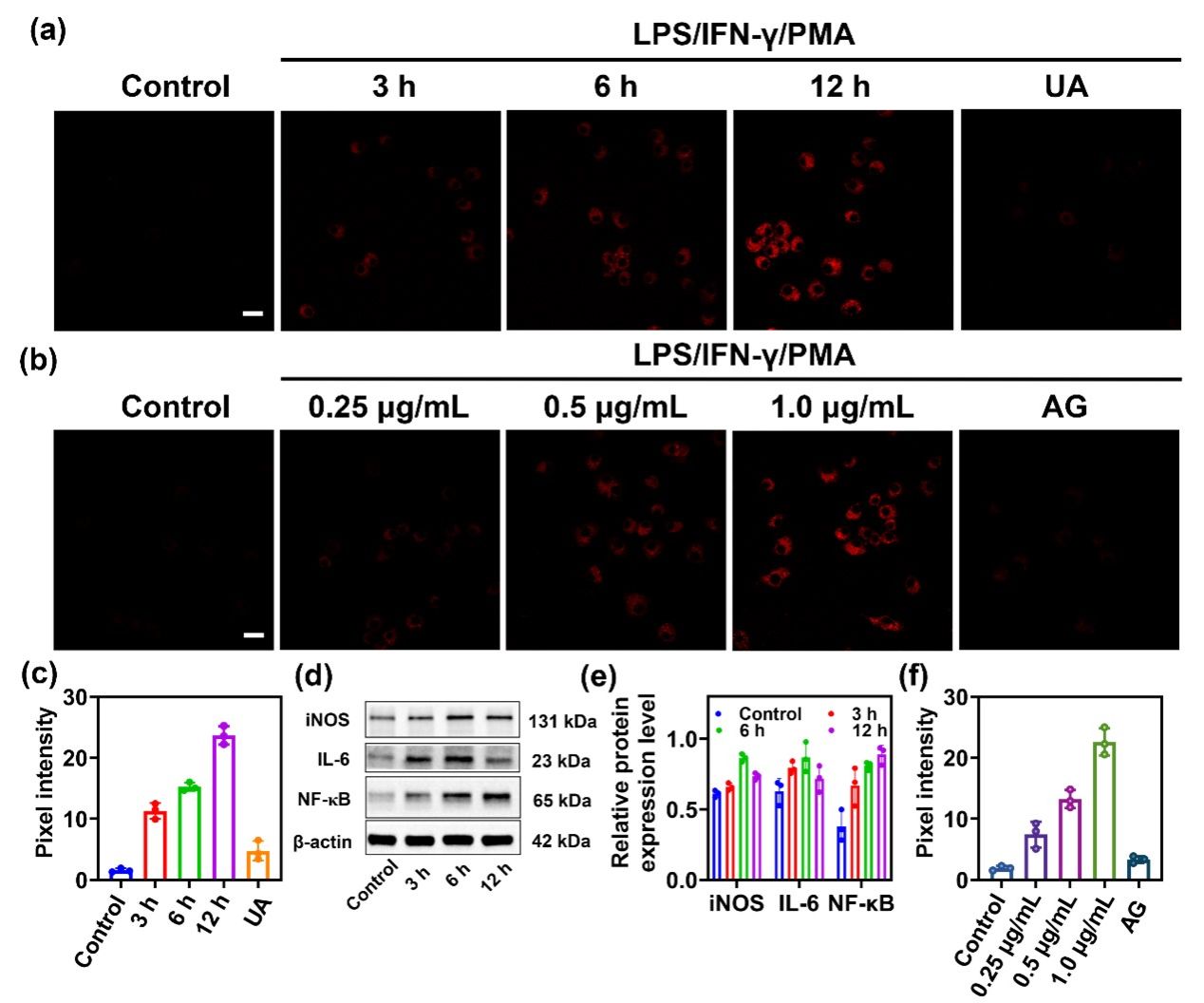
Figure 4. (a) RAW264.7 cells were pretreated with the stimulus LPS (1.0 μg/mL)/IFN-γ (100 ng/mL) for different periods (0, 3, 6, 12 h), PMA (10 nM) for 30 min or LPS (1.0 μg/mL)/IFN-γ (100 ng/mL) plus UA (200 μM) for 12 h, PMA (10 nM) for 30 min, and then incubated with DCI2F-OTf (10 μM) for 30 min. (b) RAW264.7 cells were pretreated with LPS (0, 0.25, 0.5, 1.0 μg/mL)/IFN-γ (100 ng/mL), PMA (10 nM), or LPS (1.0 μg/mL)/IFN-γ (100 ng/mL) plus AG (1 mM) for 12 h, PMA (10 nM) for 30 min, and then incubated with -OTf (10 μM) for 30 min. (c) The pixel intensity of DCI2F-OTf labeled cells in (a). (d) The protein levels of iNOS, IL-6, and NF-𝜅B were detected by western blot analysis. (e) The relative protein expression level of iNOS, IL-6, and NF-𝜅B in (c). (f) The pixel intensity of DCI2F-OTf labeled cells in (b). Error bars represent s.d., n=3. Scale bar: 50 μm.
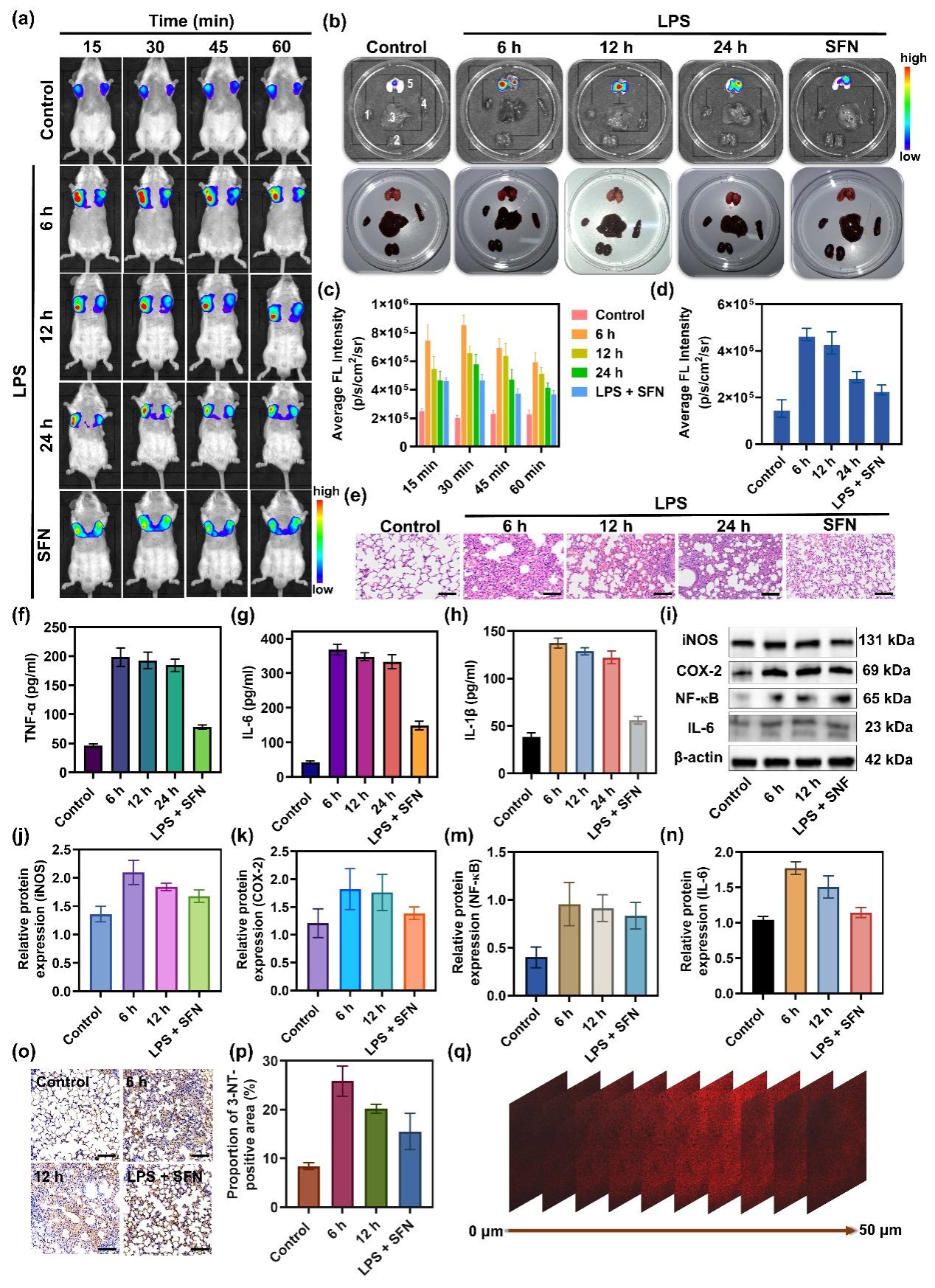
Figure 5. (a) NIRF images of control and LPS-stimulated mice after intravenous administration of DCI2F-OTf (200 μM, 45 μL). (b) NIRF imaging and photographs of dissected major organs (1: heart, 2: kidney, 3: liver, 4: spleen, 5: lung) from mice in panel (a). (c) The histogram presents the average radiant efficiency of mice in panel (a). (d) The histogram presents the average radiant efficiency of the lung in panel (b). (e) H&E staining histological images for lung tissue in control and LPS-stimulated mice. Scale bar: 50 μm. (f-h) Serum levels of inflammatory factors TNF-α, IL-6, and IL-1β in control and LPS-stimulated mice. (i) The protein levels of iNOS, COX-2, NF-κB, and IL-6 were detected by western blot analysis. (j-n) The relative protein expression level of iNOS, COX-2, NF-κB, and IL-6 in (i). (o-p) Sections of lung tissue were immunostained with an antibody that detected 3-NT. Scale bar: 50 μm. (q) NIRF images of mice fresh lung tissue sections from ALI mice in the LPS6 group. The confocal Z-axis scan images at 0, 5, 10, 15, 20, 25, 30, 35, 40, 45, and 50 µm penetration depths. λex = 488 nm; red channel: λem = 600 - 700 nm.
Conclusion
In conclusion, we have designed a novel NIRF probe, DCI2F-OTf, for fluorescence imaging ONOO- in live cells and mice model of ALI. Under physiological conditions, DCI2F-OTf was highly sensitive and selective for ONOO- and yielded a linear response to ONOO over a wide concentration range from 0 to 70 μM. The QM covalently labeled neighboring proteins released from the reaction of DCI2F-OTf with ONOO- could solve the problem of intracellular diffusion of the probe, enabling precise imaging of intracellular ONOO-. This strategy was superior to the vast majority of ONOO- probes that have been developed so far. The excellent sensing performance and NIRF emission characteristics enable DCI2F-OTf to image ONOO- levels in mice and tissues with ALI. In vivo imaging results indicated that SFN significantly down-regulated the oxidative stress level, thereby attenuating the severity of LPS-induced ALI, and could exert a protective role against ALI. Therefore, DCI2F-OTf was a valuable tool for studying the physiological function of ONOO- and was expected to facilitate the understanding of oxidative stress diseases such as ALI and the rapid screening of corresponding therapeutic drugs.

